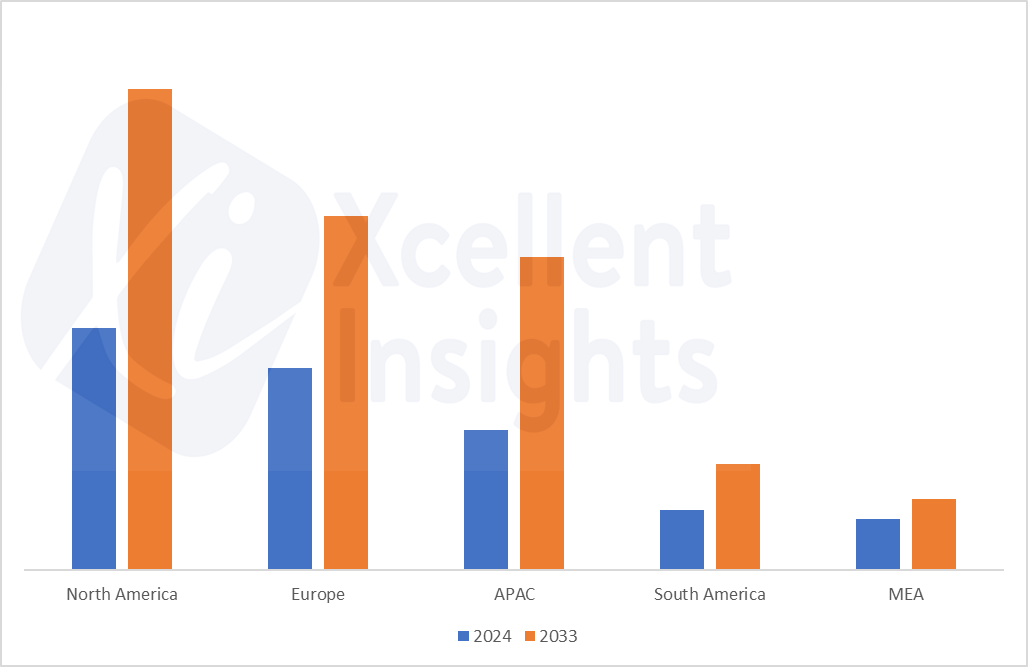
Health officials in the US recalled several hand sanitizers and aloe gels over the risk of methanol exposure. The FDA announced that 40 lots of Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholada Gel were recalled due to the presence of “alcohol denatured with methanol”. FDA stated in the notice that this methanol can be highly toxic.
The agency warned that “substantial methanol exposure” could lead to “nausea, vomiting, headache, blurred vision, coma, seizures, permanent blindness, permanent damage to the central nervous system, or death.”
The recalled items bear the labels “ARUBA ALOE Hand Sanitizer Gel 80% Alcohol made in Aruba World’s Finest Aloe” and Alcoholada Gel Pain Relieving Gel 0.5% Lidocaine Hydrochloride.”
“Although all persons using these products on their hands are at risk, young children who accidentally ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute are most at risk for methanol poisoning,” FDA said in a notice for immediate release.
The agency further said that the affected products were distributed between 01/05/2021 and 10/27/2023 and were sold in the US through the Aruba Aloe Balm N.V. website. It has also urged the existing consumers to stop using the recalled products and discard it at the earliest.